Introduction
Professor John Williams is national surgical lead at Vets Now and a world-renowned specialist in soft tissue surgery. Here he explains the investigative, treatment and follow-up care carried out in a four-month-old Border terrier emergency case in our 24/7 emergency and specialty hospital in Manchester.
Case background
Eric, a four-month-old Border terrier, presented late one night to the emergency and critical care (ECC) department of the Vets Now 24/7 emergency and specialty hospital in Manchester.
Eric had been unwell for several days before presentation in that he was not eating as well as previously and had become increasingly listless. On the day of presentation, he had not eaten at all, was hyper-salivating and was noted to have been pacing relentlessly. As the day wore on he became disorientated, and the owners reported that he was walking into furniture. At about 8pm he ‘collapsed’ and was seizuring; the owners then contacted Vets Now.
Clinical examination
At presentation to a colleague in ECC, Eric was comatose and exhibiting muscle tremors. Clinical examination revealed that he was small and underweight for his age (body condition score of 4/9), was cryptorchid, his temperature was 37.2°C, his heart rate was 120 bpm, pallor of his mucous membranes was noted.
On careful questioning by the triage nurse, it became evident that Eric had been a ‘poor doer’ since the owners had obtained him at eight weeks of age. He was fully vaccinated and had recently been ‘wormed’. They reported that his appetite was inconsistent, he would frequently show ‘odd’ behaviour including pacing round and round a room and hiding in dark areas; was subject to intermittent bouts of vomiting and diarrhoea, and they also thought he drank more water than they expected a pup to do. His episodes of ‘odd’ behaviour would frequently occur after a meal. There was no known access to toxins, and neither was trauma reported.
The ECC clinician took the view that the main concern was to stabilise Eric and formulate a definitive diagnosis later. However, from the differential diagnoses, hepatic encephalopathy was deemed to be the most likely until proven otherwise Blood was taken for analysis at the same time as management was initiated.
Stabilising the patient
Initial stabilisation was aimed at minimising the risk of airway aspiration due to Eric being comatose; propofol was drawn up at 0.5mg/kg and was administered intravenously to effect to allow intubation. This had a two-fold effect in allowing control of the airway and propofol has been shown to be beneficial in controlling some cases of hepatic encephalopathy seizures; the propofol was continued as a CRI (at 0.05-0.1 mg/g/min. As Eric was able to breathe normally at this stage, he was not ventilated, but his end tidal CO2 was monitored. Consideration was given to giving flumazenil, an intravenous benzodiazepine receptor antagonist; however, its use is still controversial and may have little real benefit unless the patient is already on benzodiazepines. It is worth noting that the ECC team did not consider using either diazepam nor midazolam, as there is some evidence that they may exacerbate seizuring in HE patients (Lidbury et al. 2016). The team did consider using the really excreted levetiracetam but the experience of using it suggest that the onset is not rapid enough for Eric’s condition.
Initial treatment
The initial blood results on Eric showed a mild hypokalaemia, a hypoalbuminaemia, a low BUN and hyperammonaemia which were consistent with HE. Based on the results Eric was given intravenous 0.95 sodium chloride with 5.5% glucose monohydrate and as supplemented with 7mmol per 250mL. Blood potassium and glucose were checked regularly while he was comatose and the potassium supplement reduced when necessary.
As the primary concern (pending results) was a severe encephalopathic crisis antibiotic was administered intravenously (Co-amoxiclav at 22mg/kg) as Eric could not be given oral therapy (to be repeated every eight hours). A 30-minute retention lactulose enema was given via a Foley catheter with three mL of lactulose mixed with 27 mL of water. Care must be taken that too much lactulose is not used as it may lead to electrolyte disturbances. Eric was written up for 50mL warm water enemas every four to six hours until oral administration of lactulose was possible. Omeprazole was given intravenously every eight hours, as there is a risk of gastrointestinal ulceration in such cases.
The full biochemistry and haematology panel showed an elevated ALT and Alk Phos and a moderate leucocytosis together with a mild microcytic, normochromic, nonregenerative anaemia. These changes indicated that further diagnostic testing would be required to drill down the diagnosis.
Eric’s propofol CRI was slowly withdrawn after 12 hours when he regained consciousness and did not show any signs of seizuring. He was initially ataxic and blind (the amaurosis persisted for a further 48 hours). Though still on intravenous fluids, the ECC clinician decided to put Eric on oral medication and to start a suitable diet to control his HE in the short to medium term. Co-amoxiclav was prescribed at 12.5mg/kg bid, and lactulose at five mL every eight hours initially. The dose of lactulose would be titrated to effect and raised or lowered as needed until Eric passed three to four soft but formed stools per day. It should be noted that neither metronidazole nor neomycin was prescribed, as metronidazole may lead to signs similar to HE and neomycin, even orally can lead to nephrotoxicity and ototoxicity. The omeprazole was also changed to oral dosing three times a day.
Diet and discharge
As diet plays a large part in precipitating HE, careful thought was given what to feed Eric. Care must be taken with protein load, but severe protein restriction is not recommended for dogs as it can cause protein malnutrition. A commercial diet that contained 14-18% protein on a dry matter basis was chosen as it also had restricted copper and sodium content, and with supplemented zinc and antioxidants. Once stable the protein content could be further increased by adding soya-based protein.
Three days after presentation, Eric was bright and alert and very active. As he was eating well, pre-and post-prandial bile acid levels were assessed. His pre-prandial level was 35mmol/L (reference range <10mmol/L) and post-prandial 160mmol/L (reference range <25mmol/L). This suggested hepatic dysfunction with a portosystemic shunt being the most likely diagnosis. Following a case conference between the ECC and surgery clinicians, it was decided that rather than investigate further at this stage, with either abdominal ultrasound or a CT scan (both would require sedation). Eric would be discharged home on a three-week course of antibiotics, lactulose and the commercial hepatic support diet.
You might also be interested in:
Returning to the clinic
After three weeks Eric returned to the clinic. He was still bright and active and had no further episodes of HE. He was sedated with and underwent a contrast CT scan this showed a portocaval PSS.
Eric was transferred to the surgical team as evidence indicates that surgery has a better long-term outcome (Greenhalgh et al. 2010; Thieman Mankin 2015). In addition to his ongoing medication, levetiracetam was prescribed at 20mg/kg every eight hours, starting 48 hours preoperatively. There is some evidence that this may help prevent post-surgical seizuring (Fryer et al. 2011).
Due to Eric’s age and diagnosis he was fed up until four hours pre-operatively. Once the anaesthetic checklists (identified patient and procedure, checked his weight and calculated drug dosages including any required for resuscitation) was completed. Eric was scheduled for blood glucose checks every 30 minutes during the procedure and until he was eating post surgically. He was premedicated with methadone, and anaesthesia was induced with intravenous propofol, titrated to effect.
Surgery
For surgery, Eric was placed in dorsal recumbency, and the abdomen was clipped from 3cm cranial to the xiphisternum to caudal inguinal area. The skin was prepped before he was taken into theatre.
In theatre, the surgical checklist was completed; this included checking swab numbers, an instrument count, and making sure that all the required instrumentation was available and working.
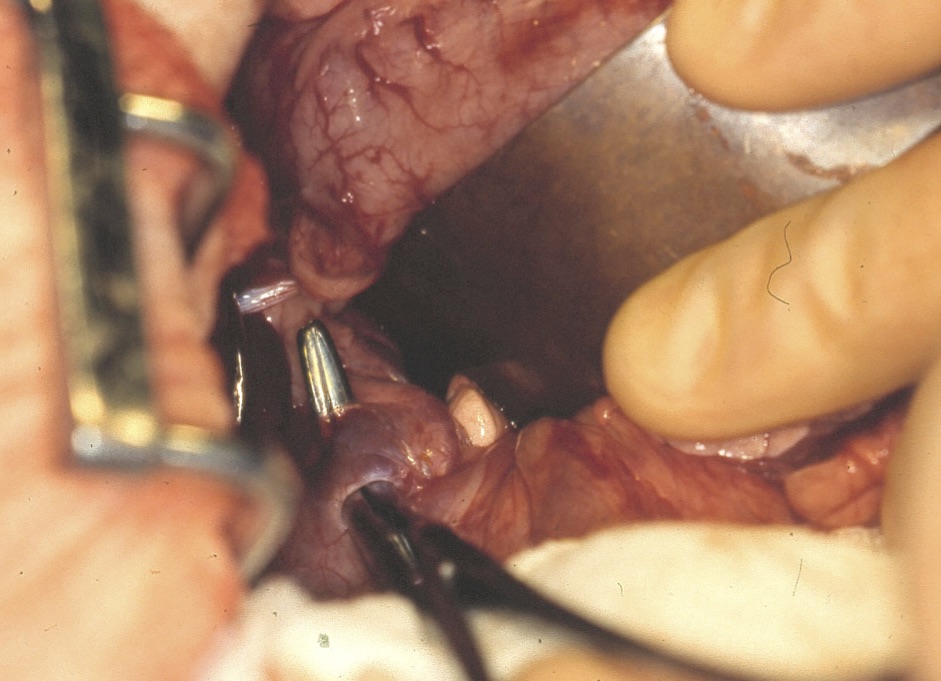
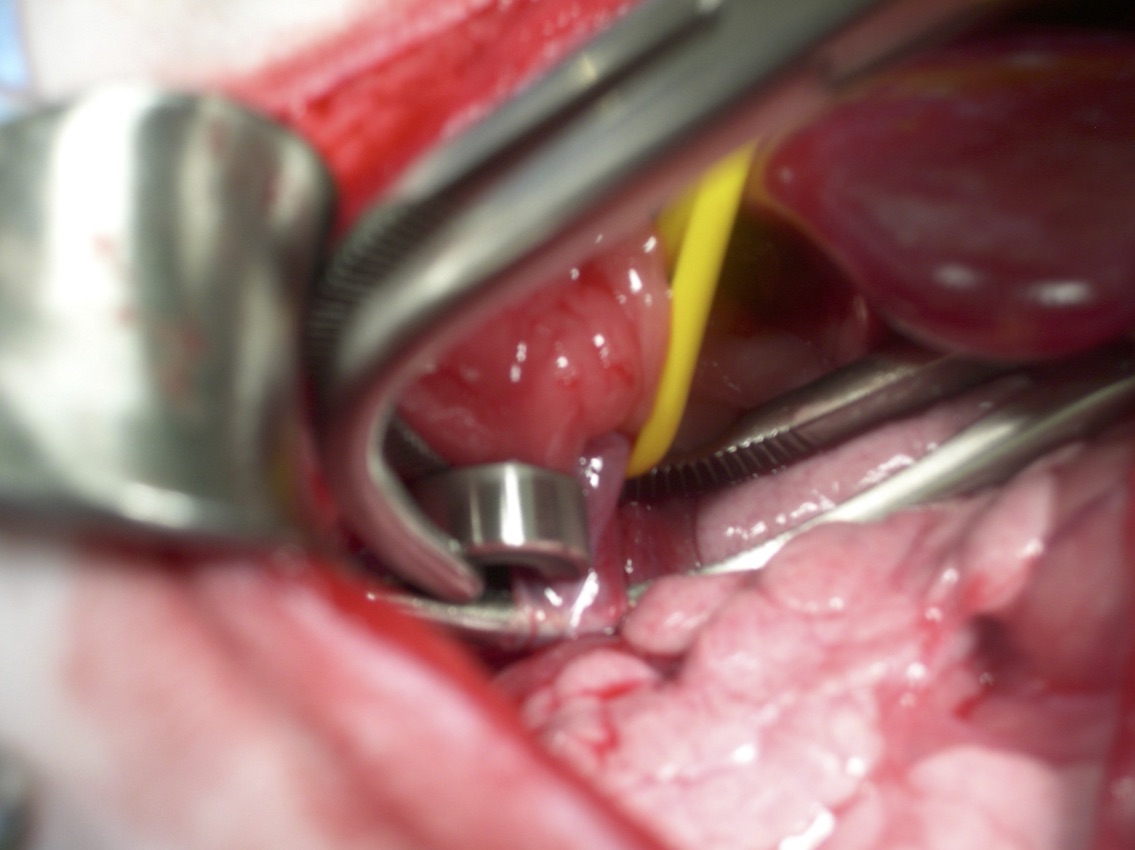
Once fully draped a midline coeliotomy was carried out, the falciform fat was excised with the aid of diathermy. The duodenal manoeuvre was carried out to view the epiploic foramen, and the portocaval shunt was confirmed at that level. The omental bursa was divided to allow improved access to the shunt vessel. Lahey’s bile duct forceps were used to bluntly dissect around the vessel as close as possible the caudal vena cava; once a ‘tunnel’ had been created, a right-angled tonsillectomy clamp was passed around the vessel.
Recovery
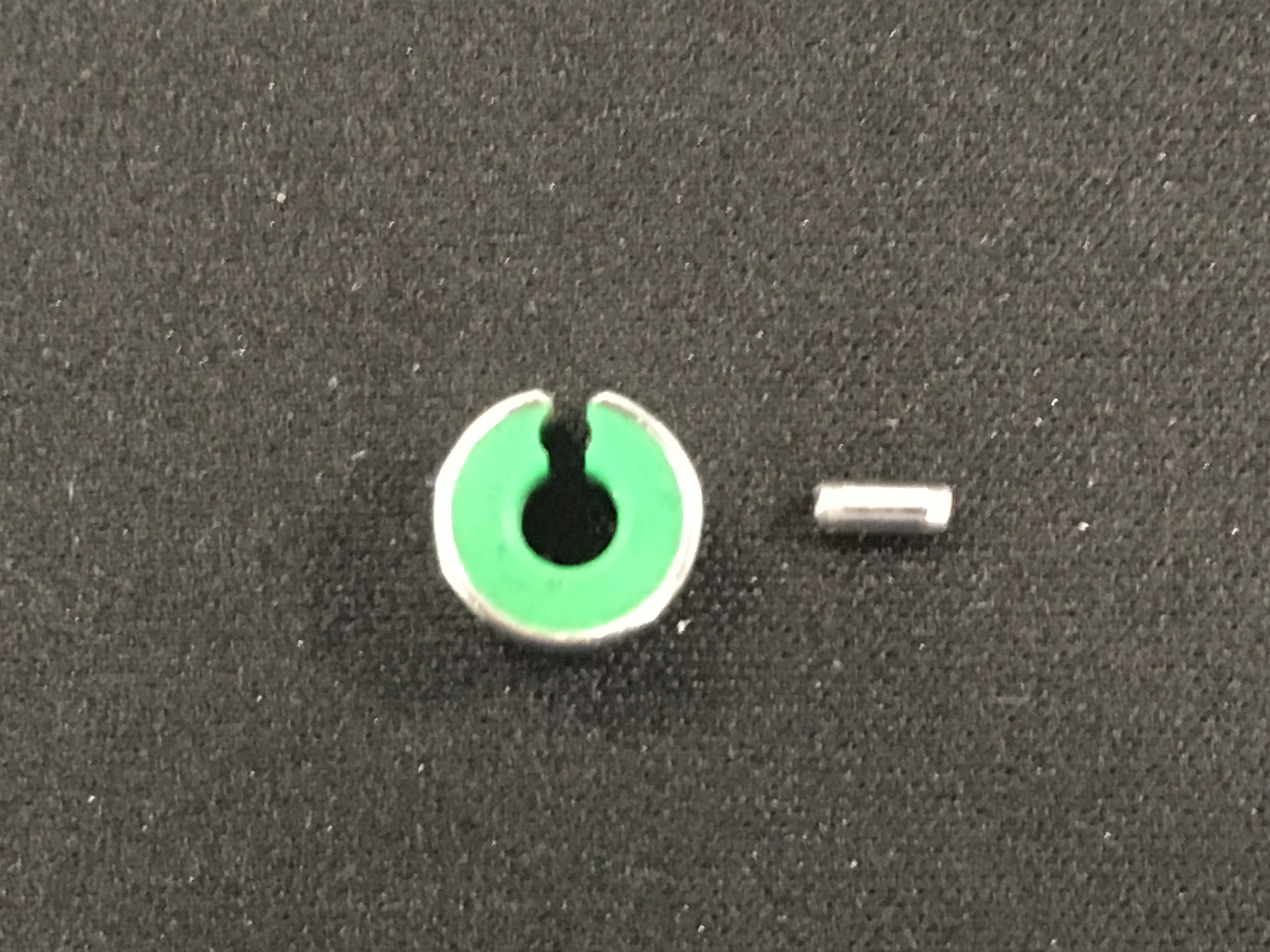
Once the constrictor was applied over the flattened shunt vessel, the tonsillectomy clamp was withdrawn. The vessel re-expanded apart from the narrowing as it passed through the AC. The circulation was allowed to equilibrate over five minutes, and there was no evidence of portal hypertension. At this point, the key was inserted into the AC to prevent it from slipping from the shunt vessel (Vogt et al. 1996).
Abdominal closure was routine with continuous three metric polydioxanone (PDSII, Ethicon) in the linea alba and a continuous horizontal mattress suture of barbed two metric 90 Glycomer 631 (V-loc, Covidien) placed intradermally. A non-adherent dressing was secured over the wound (Primapore, Smith & Nephew).
Eric made an excellent recovery from surgery and was eating well within two hours. In addition to his ongoing medications, he was pain scored, and methadone was given initially, this was changed after 12 hours to buprenorphine. He was kept in hospital for three days, to monitor for any potential post-surgical seizures.
Final discharge
Eric was discharged on a six-week course of medication and diet as the AC can take up to six weeks to attenuate the shunt vessel (Hunt et al. 2014). At his six-week check he was happy and bright and eating well, his oral medication was stopped, and the owners advised to introduce a normal diet over a two-week period. Eric is due a further check in three months time; he will not have his bile acid levels checked as there is poor correlation between post-surgical levels and outcome.
The success for Eric is reinforced by the team ethos of working in a 24/7 ECC and Specialty hospital, which allows for critical cases to be seen at any time of day or night and managed by the ECC team until it can be transferred to the necessary discipline for definitive management.
Further reading
Fryer, K.J. et al., 2011. Incidence of Postoperative Seizures with and without Levetiracetam Pretreatment in Dogs Undergoing Portosystemic Shunt Attenuation. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine.
Greenhalgh, S.N. et al., 2010. Comparison of survival after surgical or medical treatment in dogs with a congenital portosystemic shunt. Journal of the American Veterinary Medical Association, 236(11), pp.1215-1220.
Hunt, G.B. et al., 2014. Evaluation of In Vivo Behavior of Ameroid Ring Constrictors in Dogs with Congenital Extrahepatic Portosystemic Shunts Using Computed Tomography. Veterinary Surgery, 43(7), pp.834-842.
Lidbury, J.A., Cook, A.K. & Steiner, J.M., 2016. Hepatic encephalopathy in dogs and cats. Journal of Veterinary Emergency and Critical Care, 26(4), pp.471-487.
Thieman Mankin, K.M., 2015. Current Concepts in Congenital Portosystemic Shunts. Veterinary Clinics of North America: Small Animal Practice, 45(3), pp.477-487.
Vogt, J.C., KRAHWINKEL, D.J. & BRIGHT, R.M., 1996. Gradual occlusion of extrahepatic portosystemic shunts in dogs and cats using the ameroid constrictor. Veterinary Surgery, 25, pp.495-502.